Vat dye, one of the most important dye class, retains C=O chromophore in structure. This chromophore renders water insolubility. From an aqueous media, dyes are reduced and solubilized. In this state dyes show affinity for fibers.
With higher strike rate, exhaustion of vat dye is excellent. Fastness properties showed by vat dye is brilliant. With a high tinctorial power, all hues are available.
Vat dyes are somewhat different from the other type of dyes. Because they are found in insoluble form. The dyeing procedure of vat dye is not as the same as the other dyes like reactive, direct, azoic, acid or basic dyes.
You can clearly make a connection with the “Sulphur dyeing” procedure. Because their use in dyeing involves the same principle. Vat dyes in particular give dyeing on cellulosic fibers with the best overall fastness properties.
In short vat dye
Vat dyes are insoluble in water. So you can say, they act like pigment. But we called them vat dyes. Why? The reason is “with the presence of alkali, a chemical reduction process is carried out to convert the water insoluble pigment into a water soluble leuco form which has substantivity for cotton.
After dyeing with the leuco compound, oxidation process is carried out to get back the insoluble pigment form. The overall process therefore involves three key steps:
(1) Vatting process is carried out where the vat pigment is reduced to form a water soluble leuco compound.
(2) Absorption is carried out by the fiber at dyeing
(3) Oxidation is carried out so that the insoluble pigment form can be reformed inside the fiber.
The use of strongly alkaline solutions (pH 12–14) for vatting and dyeing limits the use of most vat dyes to cellulosic fibers.
Vat dye- Why so called?
‘Vat’ means vessels, natural pigment fermented in vessels, so called vat dye. The dye takes their generic name from vatting. The vat dyes are naturally obtained coloring materials from the ancient time and kept into wooden vat and make solubilize in vat by the process of fermentation.
The process of converting the insoluble vat dyes into soluble form is called vatting.
Mainly this dye is sued for cellulosic fibers but they can dye protein fibers also. This dye is extensively used for dyeing denim or jeans.
Trade names of Commercial Vat dye
| Trade name | Company | Country |
| Caledon | I.C.I | U.K |
| Solanthrene | CFMF | France |
| Cibanone | Ciba-Geigy | Switzerland |
| Mikanthrene | Mitsui Chemical Industries | Japan |
| Rodmanthrene | ACNA | Italy |
| Sandozol | Sandoz Ltd | Switzerland |
| Solasol | Francolor | France |
| Soledon | ICI Ltd | U.K |
| Algosol | General Dyestuff Corporation | U.S.A |
| Navisol | Indian Dyestuff Industries | India |
Properties of vat dyes
- Vat dyes are insoluble in water
- They can’t directly applied on the textiles
- Vatting process is required to convert the dye in soluble form
- Vatting is carried on alkaline medium
- Oxidation is required for the final color development
- Vat dyeing is carried on alkaline medium
- Can be dyed both cellulosic and protein fiber but mostly used for dyeing cellulosic fibers
- Vat dyes have excellent color fastness properties but rubbing fastness property is poor
- Vat dyes are comparatively expensive in price
- Difficult or complicated dyeing procedure
- Wash fastness is very good rating 4-5
- Sometimes, can causes skin diseases
- Practical use is limited due to the complicated dyeing procedure
- Wide range of color can be produced
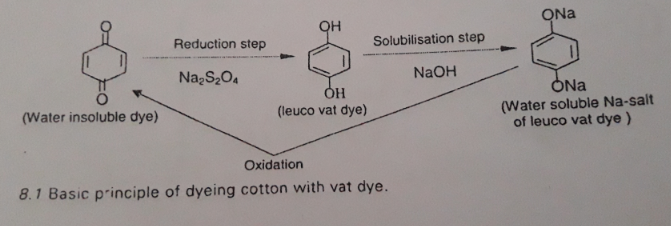
Basic Principal of Vat Dye
Vat dye is water insoluble and non-ionic. They are converted to leuco vat dyes by reduction process. The reduction process is followed by solubilisation with alkali to show substantivity to cellulose. After dyeing, parent structure is recovered by oxidation process.
So, vat dyeing generally involves the three steps:
- Reduction of vat dye
- Dyeing
- Oxidation to parent dye
Reduction of Vat Dye
Before dyeing, reduction and solubilisation of vat dye is a must. Indigo requires around (-700 to -750) mV and anthraquinoid dyes require (-800 to -1000) mV for complete reduction. It depends on the vat dye class such as IK or IK etc.
Na2S2O4 As Reducing Agent
It is the universally accepted reducing agent for vat dye. It partially reduces insoluble vat dye to leuco vat dye. Little excess of this leuco dye retains stability of reduced liquor that is actually needed for levelled dyeing. It can produce a sediment free clear reduction bath.
If you add an excess of it then it leads to over-reduction of vat dye. As a result, a huge amount of dye wastage occur.
So, how it works? For reduction of vat dye, nascent hydrogen is required. It does the exact work. When it is added to the water or alkali, it releases nascent hydrogen for reduction of vat dye. It reduces Indigo at 30-50°C and forms biphenols (leuco-indigo). To carry out dyeing at room temperature, the reduced form is quite stable.
Depending on dye class, other dyes are reduced at different temperature.
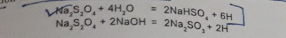
However, Sodium hydrosulphite is very unstable. No matter water, any thermal reason, oxidation is responsible for decomposition of it.
- Hydrolytic decomposition

- Thermal decomposition
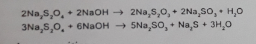
- Oxidative decomposition

- Other possible decomposition
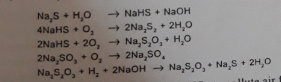
The amazing fact about Na2S2O4 is that it can generate (-700 to -1000 mV). This enables it for varied reduction potential. It can be achieved by changing its concentration, alkali or temperature.
Other reducing systems are also available:
>Wooden vat or copperas method
>Zinc-lime method
>Sodium borohydride method
Over Reduction of Vat Dye
If too much of sodium hydrosulphite is added to the bath or the vatting temperature is not properly maintained, over reduction may take place. Depending on dye structure, over reduction may be of different types:
Simple over-reduction
Simple over reduction is the result of either high concentration of Na2S2O4 or too high temperature.
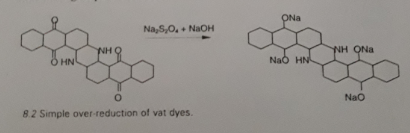
Hydrolysis
At high temperature, benzyl amino or acyl amino group containing vat dyes are hydrolysed. This can also take place for prolonged vatting time. This results in duller and less fast shades.
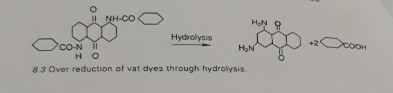
De-halogenation
Halogenated dyes such as violet 2R or violet 3R loses their halogen while the temperature gets too high. It results in poor bleaching.

Molecular re-arrangement
Over 66°C, certain vat dyes such a khaki 2G or olive T can experience a permanent change in hue. This results from irreversible change in some keto groups.
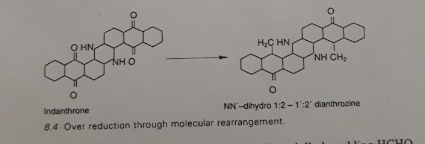
By adding HCHO or glucose in the bath, we can reduce the chance of over reduction.
Solubilization of Vat dye
With NaOH, reduced leuco vat is converted to its sodium salt. This helps it to become water soluble and develop affinity for cellulose.
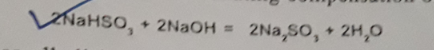
For Vat dye, NaOH is a popular solubilizing agent. Again it has multi-functional properties. Here is the list:
>Dissolves leuco dye into its sodium salt
>Develops affinity for cellulose
>Neutralizes acidic by products
>Maintains pH of bath
>Suppresses hardness of water
Oxidation of Vat Dye
Oxidation is carried out to restore original dye structure and to trap the dye molecules inside the fiber. It is done to develop actual shade. But make sure a thorough wash is given to the fabric to remove even the last trace of alkali.
Oxidation can be carried out by –
>Hydrogen per-oxide at 50-60°C
>Sodium perborate at 50-60°C
>Sodium hypochlorite at room temperature for 15 to 20 minutes
>In an open air
Air oxidation is suitable for smaller batch and loose structures. Compact structures are oxidized by chemical. It is carried out properly otherwise patchy dyeing may take place.
Oxidation must be carried at lowest possible temperature for minimum time otherwise cotton may get oxidized. Among of all, hydrogen per oxide is universally chosen for oxidation.
Garments Dyeing Process With Vat Dye
Vat dye produce a brilliant shade in cellulosic fibers. Garments can be easily dyed by using vat dye. If you are interested to know about garment dyeing process with perfect recipe using vat dye, you can check the following article.
Recommended read: Chemical Constitution of Vat Dyes
Photo-chemical Degradation
Few yellow, orange and brown vat dyes show deterioration in strength of color. On prolonged light exposure, a fading effect is experienced on shade. With the presence of metal in cellulose, a slower decomposition may observed due to the following reasons:
>Absorbed sunlight
>atmospheric oxygen
>Moisture

Dyeing Defects
Various dyeing faults are observed. They are enlisted below:
>Dark selvedge
>Light spot
>Oxidation mark
>Streaky dyeing
>Poor rubbing fastness
Stripping of Vat dye
If desired shade is not achieved or uneven dyeing takes place, it needs to correct them by partial or complete stripping. Vat dyes produce very fast shades. They exhibits a strong affinity for cotton also. As a result, complete extraction is not practiced.
Stripping may be carried in two ways:
>Partial stripping
>Complete stripping
Partial stripping
Na2S2O4 and NaOH is used at a specific temperature to treat the dyeings. This reduction is carried out for 30 minutes for levelling of shade as well as partial stripping. To promote the rate of levelling, addition of a non-ionic surfactant is practiced.
Complete stripping
For complete stripping, the method of partial stripping is carried out with a cationic surfactant. In this process, complexes are formed with reduced and solubilized anionic dye and lose affinity for cotton and comes out from dyeings.
In hot DMSO, most of the vat dyes are soluble. So, dyes can be easily extracted with it.
Last thought
Vat dyeing is really an interesting process. The popularity of jeans or denims make this dye more popular. You can also check: Chemical constitution of vat dye
Salma Hasin the author of this site completed her BSc. in Textile Engineering (Wet Processing Engineering). She wants to share her knowledge to help students in their studies and businessman & entrepreneurs in their businesses in making wise decisions fast.

