Reactive dyes are widely used dyes in our country. They have reactive group in their structure which can create covalent bond with the textile fibers. They are very popular dyes in terms of excellent brilliancy, wide range of shades, overall good fastness properties.
Reactive dye is used not only for textile dyeing, it is also used for hair dyeing or decorative purpose.
Reactive digital printing – yes, you heard right! Conventional printing using reactive dyes are pretty common for us. But now technology becomes very smart and inkjet printers use reactive dyes for permanent and eco-friendly printing. It creates perfect digital artwork on your tees.
Properties of Reactive Dye
- Reactive dye is anionic in nature
- Reactive dye is water soluble dye
- They can be found in powder, liquid or paste form
- They create covalent bond with the textile fibers
- They are extensively used for dyeing cellulosic fabrics
- They can also be used for dyeing protein fibers
- A certain amount of dye is hydrolyzed during dyeing ( 15-40% )
- They have good light fastness ( rating 6 )
- They have excellent wash fastness ( rating 4-5 ) as they attach to the fibers with covalent bond ( a strong chemical process )
- They have moderate rubbing fastness
- Dyeing procedure and Fixation is occurred in alkaline condition
- Huge electrolyte is required for dyeing process
- Comparatively cheap
- Wide range of shades with excellent brilliancy can be produced with them
- They have better substantivity and reproducibility
Classification of Reactive dye
Reactive dyes can be classified according to reactive group, reactivity, chemical constitution, temperature etc. All the types have discussed below!
Depending on reactive group
Depending on the reactive group, reactive dye is of two types. They are:
1. Halogen (commonly chlorine) derivatives of nitrogen-containing heterocycle
Triazine group : Example: Procoin, Cibacron


Fig: Triazine group Fig: Dichlorotriazine
Pyrimidine: Example: Reactone

Fig: Trichloro pyrimidine
Quinoxaline

Fig: Levafix
2. Activated vinyl compounds
Vinyl sulphone: remazol
Vinyl acrylamide: primazine
Vinyl sulphonamide: levafix.
Depending on chemical constitution
Depending on chemical constitution reactive dye is of three types. Here they are:
1. Chloro triazine Reactive dyes
- Monochloro dyes.

- Dichloro/Bifunctional dyes

- Trichloro dyes
2. Vinyl Sulphone dyes

3. Heterocyclic Halogen containing dyes

Fig: Levafix E
Depending on Reactivity
- Low Reactive dye: pH maintained= 12-12.5 Alkali used – NaOH
- Medium reactive dye: pH maintained= 11-12 Alkali used – NaCO3
- Highly reactive dye: pH maintained= 10-11 Alkali used – NaHCO3
Depending on Temperature
- Cold Brand dyes: Dyes of these brand has reactive group of high reactivity thus dyeing can be possible at lower temperature even at 33 -60
For example: PROCION M, LIVAFIX E.
- Medium Brand dyes: Dyes of these brand has reactive group of moderate reactivity. Dyeing can be possible at 60-71℃
For example, Remazol, Livafix
- Hot Brand dye: Dyes of these brand has reactive group of least reactivity thus dyeing procedure needs high temperature ( 72-93℃ )
For example PRICION H, CIBACRON
Recent Classification of Reactive dyes
(1) Alkali-controllable dyes: Alkali-controllable dyes have relatively high reactivity. These dyes have moderate substantivity towards substrate. Low temperature is enough for the application of these dye. For fixation, a careful alkali adition is required. Examples include DCT, DFCP and VS reactive dyes
(2) Salt-controllable dyes: Salt-controllable dyes required high temperature close to 80 °C as the reactivity of these dyes is quite low for cotton even under the alkaline condition. They have appreciable substantivity. Here salt addition facilitates level dyeing by promoting exhaustion. Examples in this class include TCP, MCT as well as MFT reactive dyes
(3) Temperature-controllable dyes: These dyes undergo fixation at high temperatures even under neutral conditions. The NT dyes are in this class.
We already come to know about the classification of reactive dye depending on the reactive group present in it. Let’s know elaborately about reactive group present in the reactive dye.
Classification of Reactive Group
Reactive dyes have reactive groups in their structure. Giles classified reactive groups into five categories depending on their mechanism of reaction with the substrate.
Here’s the types:
- Nucleophilic substitution
- Nucleophilic addition
- Multiple addition-elimination reactions
- Fixation under acidic condition
- Dyes forming disulphide bonds
Let’s know the types elaborately. Here you go!
Nucleophilic Substitution
- Chlorotriazines are the most vital member of this group. By reacting the dye chromogens (that have –NH2 groups) with cyanuric chloride. Cyanuric chloride is a white solid. Its melting point is 146°C and has a pungent odor. You can say, this is a highly reactive substance available at low cost.
- Neucliophilic groups of substrate (-OH of cellulose or –NH2 of protein) reacts with chlorine atoms on the heterocyclic ring under favorable condition. This may occur by neucliophilic addition-elimination or substitution mechanism.
- Firstly, the functional group of the fiber is added to the dye molecule and then eliminates the chlorine atom. The reaction of DCT dyes occur at 20°C whereas the temperature requirement for MCT dyes is 70°C.
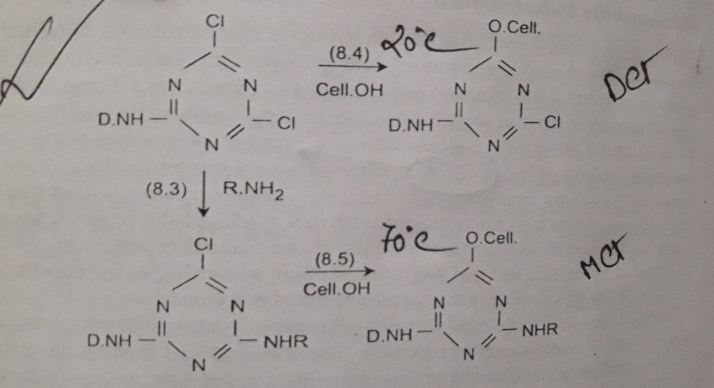
Image Source: Textile Preparation & Dyeing
The other group members are as follows:
- 2,4,5 trihalogen-pyrimidine such as Reactone (Geigy), Drimrene (Clariant), Drimalan F (Clariant)
- 2-methylsulphonyl1-4-methyl1-5-chloro-pyrimidine such as Levafix P (Bayer)
- 3,6-dichloropyrazine such as Solidazol
- 2,3-dichloroquinoxaline such as Levafix E (Bayer), Cavalite
- 2-chlorobenzthaiazol such as Reatex
- 4,5-dichloropyridazone such as Primazin
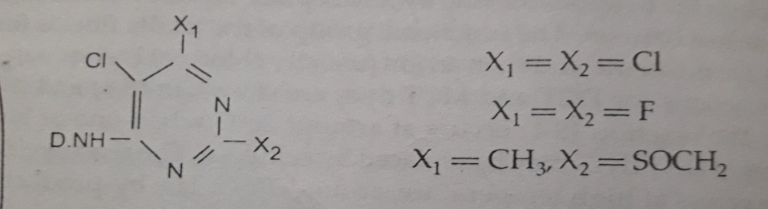
Image Source: Textile Preparation & Dyeing
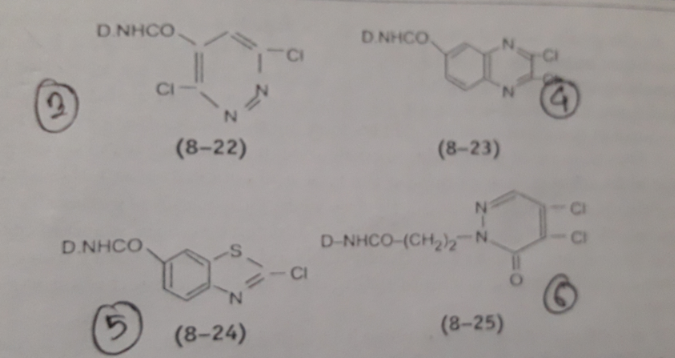
Image Source: Textile Preparation & Dyeing
Fig: 3,6-dichloropyrazine (8-22) 2,3-dichloroquinoxaline (8-23) 2-chlorobenzthaiazol (8-24) 4,5- dichloropyridazone (8-25)
Nucliophilic Addition
- Chlorotriazines or Chloropyrimidine reactive dyes are attached to the fibers by nucleophilic substitution mechanism. In this case, the compounds that form with cellulose possess the properties of esters (R-CH-O.alkyl).
- But the reactive dyes that follows nucleophilic addition mechanism for dye-fiber reaction, possess the properties of ether (R.O=C-O.alkyl) rather than ester.
- The ether linkage is formed between the azo compound and the cellulose. It can be achieved by the direct reaction of sulphuric ester or by desaturation and subsequent addition. But this type of reactions proceed far too late without any presence of activating group. Well, in this case, various electron-attracting groups are introduced for activation, the most common being sulphonyl!
- As in vinylsulphone dyes, it needs to interpose such groups between a chromophore and olefic group so that the double bond becomes polarized enough for the nucleophilic addition reaction.
- Under mild alkaline condition, such reactions proceed easily with alcohols. But it is not necessary that the vinyl sulphone groups to be present in the dye molecules.
- Various reactive group present in the commercial reactive dyes which undergo similar addition reactions. They are listed below:
- Groups yielding vinyl sulphone can be represented as:
- –SO2CH2-CH2-X
- Here, X = -OSO3H, Remazol and Remalan (Hoechst) dyes
X = -N(CH3)CH2-CH2-SO3H, Hostalan (Hoechst) dyes
X = -N(NH3+)CH2-CH2-SO3–) Remazolan (Hoechst) dyes
- Groups yielding acrylamide can be represented as:
- –NH-CO-CH2-CH2-X
- Where, X = -OSO3H or X = -Cl, Primazin (BASF)
Multiple Addition – Elimination Reactions
Some dyes react through several addition-elimination steps with the substrates functional group. Lanasol (Ciba) dyes having alpha-chloroacrylamide group, belong to this group.
Fixation under acidic condition
- We all know that reactive dyes are applied in alkaline medium on cellulosic materials. The ambition for combining dyeing and finishing, especially the resin finishing, resulted in the invention of acid-fixable reactive dyes.
- Calcobond (Cyanamid) dyes marketed by American Cyanamid Company in 1956 is such type of dye. They contain N-metylol groups obtained by reacting dyes containg melamine groups with formaldehyde.
- In 1977, ICI resulted in the introduction of Procion T (ICI). These dyes can be applied under mild acidic (pH 5-6) medium on cellulose. They are also sold as admixture with Disperse dyes under the Procilene trademark for application on polyester/cotton blend.
Dyes Forming di-sulphied bonds
Thiosulphate dyes having one or more –S.SO3H groups belong to this class. Some usual types are:
- Dyes having –S.SO3H groups directly substituted in aromatic nuclei, such as Dykolite (Southern Dyesuff Corporation) and Hydrosol (Casella).
- Dyes having –S.SO3H groups attached to the aromatic nuclei through an alkyl group, usualy –CH3 such as Inthion dyes (Hochest).
- Dyes having substitution –SO2.NH.CH2CH2-S.SO3H, often known as Bunte Salt dyes.
Fixation of such dye involves formation of an insoluble di-sulphied (Poly-condensation) bonds with fibers.
Wrapping It Up!
Reactive dye class plays an important role in our textile industry while we think of dyeing cellulose fibers. Nowadays, reactive dye printing is also developed in digital printing machine which is another great invention for all of us.
Salma Hasin the author of this site completed her BSc. in Textile Engineering (Wet Processing Engineering). She wants to share her knowledge to help students in their studies and businessman & entrepreneurs in their businesses in making wise decisions fast.

Nice explanation on reactive dyes. Thanks
Excellent post. So exciting!!!!
https://advancetextile86.blogspot.com
Very nice.
https://advancetextile86.blogspot.com