Acid dyes are mostly sulphuric or carboxylic acid salts and are essentially applied from an acidic bath and hence the name “acid dye”. Acid dyes have poor wash fastness but light fastness is quite good. Dyes possess affinity for protein fibers and mainly used to dye wool, silk and nylon.
Mechanism of Acid Dye
Dye dissolution produces a colored anion

Under acidic condition, protein and polyamide fibers achieve cationic sites. As the amount of acid absorbed by the fiber increases with time, the more and more cationic sites are introduced under strong acidic condition. The dye anions then get attached with these cationic sites. This attachment between the dye anion and fiber takes place through hydrogen bond, van der waal’s force and ionic bond.
In case of wool acid dye mechanism
Wool retains –NH2 and –COOH groups at either end of its structure which is able to make chemical reaction. Wool can be represented as: H2N- W- COOH where W retains the rest part of wool structure.
When wool is immersed in water, the H atom of carboxylic group of wool gets attached to the –NH2 group. Thus, wool chain acquire opposite electrical charges called zwitter ions:
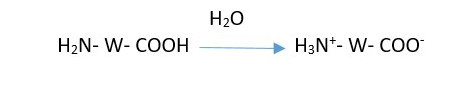
At isoelectric point, it carries no net charge. As in this point there remains equal amount of positively charged ammonium groups and negatively charged carboxyl anions. For wool, the isoelectric region is between pH 4.8 to 7.
When acid is added to this bath, it releases hydrogen ion which is taken up by the negative carboxylate ion of that zwitter ion containing wool and are transformed to electrically neutral carboxylic acid group. Thus the positively charged ammonium groups are left available which act as dye sites for acid dye. At the same time, acid anions released by the acid are absorbed by the positively charged amino ends of the keratin macromolecules.
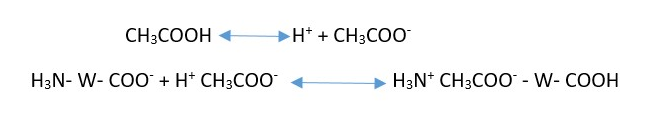
Amount of acid absorbed by the wool increases with acid treatment and generates more and more positive sites in wool. When the acid dye is added to the bath containing cationized wool, the dye anions get attached with cation of wool through electrostatic force with liberation of salt.

Some of the group in the dyes are attached to the wool by hydrogen bonds and van der waal force. As dye anion is strongly held on the protein molecules than the acetate anion, the positive sites take up the acetate ions prior to addition of dye. As further dyeing procedure proceeds, ion transfer from wool to solution and solution to wool is continued.
This is the basic mechanism of acid dye in case of wool. Let’s know the criteria for better dye uptake for acid dyes.
Dye uptake depends on the nature and concentration of acid used. More the concentration of acid in bath, more the exhaustion of dye takes place. H3PO4 is recommended over H2SO4 for dyeing of wool. 1% H2SO4 = 2% H3PO4.
H3PO4 is suitable for wool because it causes less damage of wool as well as any blends of cellulosic fibers. It also produces better shades even when buffered with Na2CO3.
Acid dyes possess both the hydrophobic or non-polar and hydrophilic or polar characteristics. The hydrophobic part presents head and the hydrophilic part presents polar tail (-COOH, -SO3H).
Whereas, wool has a hydrocarbon or non-polar backbone of keratine. It feels higher attraction for the non-polar head of dye. This is the reason for higher affinity of acid dyes for wool; dyeing is thus analogous to extraction of non-polar solvent from an organic solvent.
The funny thing is, if you introduce a non-polar group in the dye structure such as –CH, it probably increases the affinity of wool for acid dyes. On the other hand, you can reduce the affinity of wool for acid dye with the introduction of polar group (-COOH, -SO3H) in the dye structure.
With the addition of electrostatic bond like NH3+D–, the dye anion is attached to wool with co-ordinate linkage. As a result, the strength is increased. As it converts from equalizing dyes to aggregated dyes. This causes improved fastness.
Influence of dyeing parameters
Electrolyte, acid, temperature all of these parameters have influence on the dyeing rate of acid dye. The influence of these parameters are discussed below:
Effect of Electrolyte
Electrolytes act mainly as retarding or levelling agent for strong acid dyes. For super milling dyes, which are applied from a neutral bath, salt plays an opposite effect. In this case, salt promotes dye uptake on protein fibers by reducing zeta potential as wool probably acquires a negative electrical potential in neutral solution.
Effect of Acid
Levelling and milling acid dyes are not taken up by protein fiber unless acid is added to bath. Addition of acid makes the fiber cationic in nature through attachment of H atom of acid to the COO– ion making it COOH. In the absence of acid in bath, marked by pH at or above 7.0 will cause repulsion of more dye anions with little dye uptake. The rate of exhaustion is dependent upon the acidity of the bath or pH of the bath.
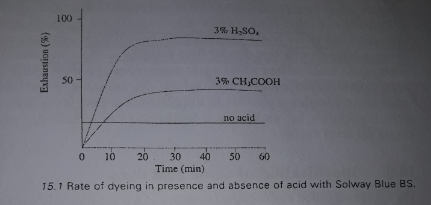
Image Source: Fundamental & Practices in Textile Coloration
Effect of Temperature
The below reaction is not possible at room temperature but only when the temperature of the bath is elevated that helps in the acceleration of dye molecules. It helps to generate required momentum for the dye molecules. If dyeing begins at 40℃ optimum results can be achieved, then the temperature raised slowly to boil and dyeing is further carried out at boil for desired time. Acid dyes are not transferred from the bath below 39℃.

To Wrap Up!
Acid dyes have wet and light fastness ranging from poor to excellent. Fastness property varies with chemical constitution as well as application class of dyes. Acid dyes exhaust well. No bleeding or fading occurs with washing. Of all the colorants, acid dyes produce the most luscious colors.
You can also check: Acid dye for nylon [An Overview]
Salma Hasin the author of this site completed her BSc. in Textile Engineering (Wet Processing Engineering). She wants to share her knowledge to help students in their studies and businessman & entrepreneurs in their businesses in making wise decisions fast.

